Generic name: dantrolene sodium
Dosage form: injection, powder, for solution
Drug class:Skeletal muscle relaxants
Medically reviewed by Drugs.com. Last updated on Feb 1, 2022.
On This Page
Dantrolene Injection Description
Dantrolene Sodium for Injection, USP is a sterile, non-pyrogenic, lyophilized formulation. Dantrolene Sodium for Injection, USP is supplied in 100 mL vials containing 20 mg dantrolene sodium, 3,000 mg mannitol, and sufficient sodium hydroxide to yield a pH of approximately 9.5 when reconstituted with 60 mL sterile water for injection USP (without a bacteriostatic agent).
Dantrolene sodium is classified as a direct-acting skeletal muscle relaxant. Chemically, dantrolene sodium is hydrated 1-[[[5-(4-nitrophenyl)-2furanyl]methylene]amino]-2,4-imidazolidinedione sodium salt. The structural formula for the hydrated salt is:
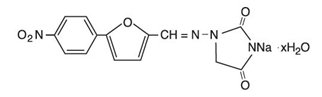
The hydrated salt contains approximately 15% water (3.5 moles) and has a molecular weight of 399. The anhydrous salt (dantrolene) has a molecular weight of 336.
Dantrolene Injection - Clinical Pharmacology
In isolated nerve-muscle preparation, dantrolene sodium has been shown to produce relax...



