DentiPatch
Generic name:lidocaine
Dosage form: Transoral Delivery System
Drug classes:Group I antiarrhythmics, Local injectable anesthetics
Medically reviewed by Drugs.com. Last updated on Jun 21, 2021.
On This Page
DESCRIPTION
The DentiPatch® system contains a local anesthetic agent to be applied topically to the oral cavity. See INDICATIONS for specific uses.
Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, and has the following structural formula:
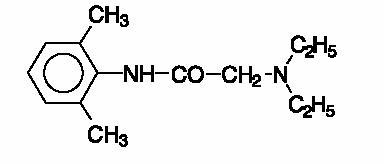
The molecular formula of lidocaine is C14H22N2O. The molecular weight is 234.34.
Each 2 cm2 patch contains lidocaine base as the active ingredient in the amount of 46.1 mg. Non-active ingredients include: karaya gum, glycerin, dipropylene glycol, lecithin, propylene glycol, aspartame, spearmint flavor, polyester film laminate and polyester-rayon fabric.
Each unit is sealed in a paper polyethylene-foil pouch.
CLINICAL PHARMACOLOGY
Mechanism of action:
The DentiPatch® system is applied to the buccal mucosa to provide topical anesthesia by releasing lidocaine. Lidocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic action.
Onset and duration of action:
The DentiPatch® system acts on intact mucous membranes to produce local anesthesia.
DentiPatch® (Lidocaine Transoral Delivery System)
Anesthesia occurs usually within 2.5 minutes of application, is present for the duration of a 15 minute application period, and persists for approximately 30 minutes following removal.
Hemodynamics:
Excessive blood levels may cause changes in cardiac output, total peripheral resistance, and mean arterial pressure. These changes may be attributable to a direct depressant effect of the local anesthetic agent on various components of the cardiovascular system.
Pharmacokinetics and Metabolism:
Absorption
While systemic availability is not an objective when lidocaine is admini...



