Deramaxx
Generic name: deracoxib
Dosage form: FOR ANIMAL USE ONLY
On This Page
Chewable Tablets
For Oral Use in Dogs Only
Do Not Use in Cats
Caution:
Federal Law (U.S.) restricts this drug to use by or on the order of a licensed veterinarian.
Description:
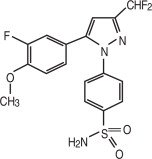
Deramaxx (deracoxib) is a non-narcotic, non-steroidal anti-inflammatory drug (NSAID) of the coxib class. Deramaxx tablets are round, biconvex, chewable tablets that contain deracoxib formulated with beefy flavoring. The molecular weight of deracoxib is 397.38. The empirical formula is C17-H14-F3-N3O3-S. Deracoxib is 4-[3-(difluoromethyl)-5-(3-fluoro-4-methoxyphenyl)-1H-pyrazole-1-yl] benzenesulfonamide, and can be termed a diaryl substituted pyrazole. The structural formula is:
Clinical Pharmacology
Mode of Action:
Deramaxx tablets are a member of the coxib class of non-narcotic, non-steroidal, cyclooxygenase-inhibiting anti-inflammatory drugs for the control of postoperative pain and inflammation associated with orthopedic and dental surgery and for the control of pain and inflammation associated with osteoarthritis in dogs.
Data indicate that deracoxib inhibits the production of PGE1 and 6-keto PGF1 by its inhibitory effects on prostaglandin biosynthesis.1 Deracoxib inhibited COX-2 mediated PGE2 production in LPS-stimulated human whole blood.2
Cyclooxygenase-1 (COX-1) is the enzyme responsible for facilitating constitutive physiological processes (e.g., platelet aggregation, gastric mucosal protection, renal perfusion).3 Cyclooxygenase-2 (COX-2) is responsible for the synthesis of inflammatory mediators.4 Both COX isoforms are constitutively expressed in the canine kidney.5 At doses of 2-4 mg/kg/day, Deramaxx tablets do not inhibit COX-1 based on in vitro studies using cloned canine cyclooxygenase.6 The clinical relevance of this in vitro data has not been shown.
Although the plasma terminal elimination half-life for Deramaxx tablets is approximately 3 hours, a longer duration of clinical effectiveness is observed.
Summary pharmacokinetics of Deramaxx tablets are listed in Table 1.