DeramsilkRx Anodynexa Pak
Generic name: diclofenac sodium delayed release tablets, ranitidine tablets, capsaicin cream
Dosage form: kit
Diclofenac Sodium Delayed-Release Tablets USP
Rx only
Prescribing Information
On This Page
Cardiovascular Risk
- NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk. (see WARNINGS.)
- Diclofenac sodium delayed-release tablets is contraindicated for the treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).
Gastrointestinal Risk
- NSAIDs cause an increased risk of serious gastrointestinal adverse events including inflammation, bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events. (see WARNINGS.)
DESCRIPTION
Diclofenac, as the sodium salt, is a benzene-acetic acid derivative. The chemical name is 2-[(2,6-dichlorophenyl)amino] benzeneacetic acid, monosodium salt. The molecular weight is 318.14. (Its molecular formula is C 14H 10Cl 2NaO 2, and it has the following structural formula:
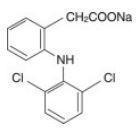
Each enteric-coated tablet for oral administration contains 75 mg of diclofenac sodium. In addition, each tablet contains the following inactive ingredients: aluminum hydrate, colloidal silicon dioxide, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, polyvinyl acetate phthalate, propylene glycol, silica, sodium alginate, sodium starch glycolate (Type A), stearic acid, synthetic black iron oxide, talc, and titanium dioxide.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Diclofena..



