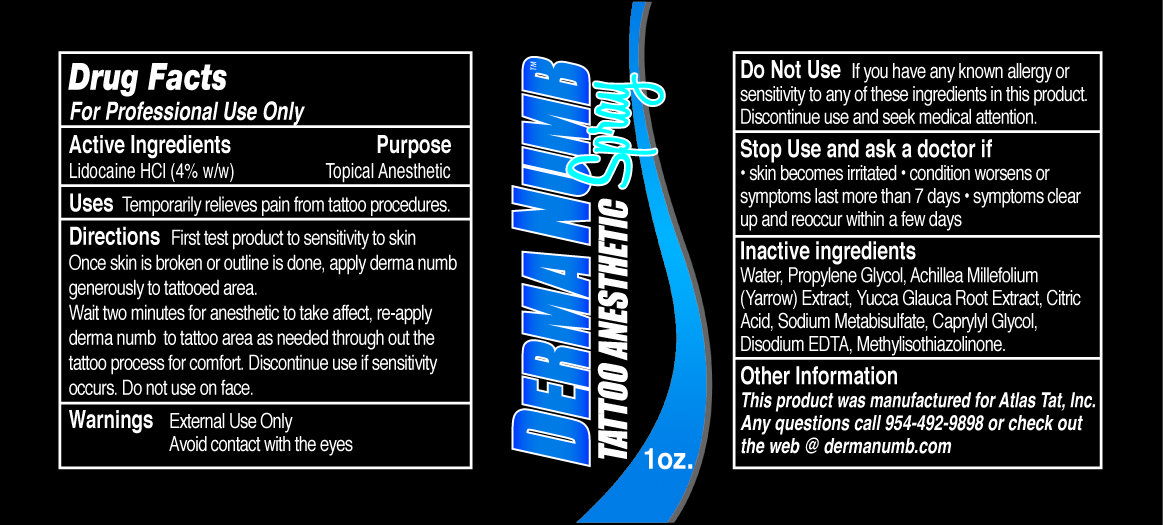
Derma Numb Spray
Generic name:lidocaine hydrochloride
Dosage form: topical spray
Drug class:Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Active Ingredients
Lidocaine HCI
Purpose
Topical Anesthetic
Uses
Temporarily relieves pain from tattoo procedures.
Directions
First test product to sensitivity to skin.
Once skin is broken or outline is done, apply derma numb generously to tattooed area.
Wait two minutes for anesthetic to take affect, re-apply derma numb to tattoo area as needed through out the tattoo process for comfort. Discontinue use if sensitivity occurs. Do not use on face.
Warnings
External Use Only
Avoid contact with the eyes
Do Not Use
If you have any known allergy or sensitivity to any of these ingredients in this product. Discontinue use and seek medical attention.
Stop Use and ask a doctor if
• skin becomes irritated • condition worsens or symptoms last more than7 days • symptoms clear up and reoccur with a few days
Inactive ingredients
Water, Propylene Glycol, Achillea Millefolium (Yarrow) Extract, Yucca Glauca Root Extract, Citric Acid, Sodium Metabisulfate, Caprylyl Glycol, Disodium EDTA, Methylisothiazolinone.
Other Information
This product was manufactured for Atlas Tat, Inc.
Any questions call 954-492-9898 or check out the web @ dermnumb.com
| DERMA NUMB lidocaine hci spray | |||||||||
| |||||||||



