DermacinRx Lidotral
Generic name:lidocaine hydrochloride
Dosage form: cream
Drug class:Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Jan 24, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
DermacinRx Lidotral Description
Lidotral™ (Lidocaine HCl) 3.88% Cream contains Lidocaine HCl 3.88% in a mild acidic vehicle. Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), and has the following structure:
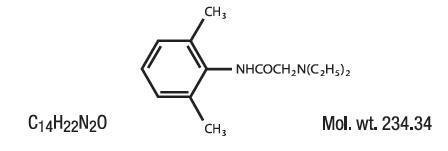
Ingredients: Each gram of Lidotral™ 3.88% Cream contains Lidocaine HCl USP 38.8 mg. Inactive Ingredients include: Calcium Acetate, Ceteareth 20, Cetearyl Alcohol, Glycerin, Methylparaben, Mineral Oil, Petrolatum, Propylene Glycol, Propylparaben, Purified Water, Sodium Phosphate Monobasic.
DermacinRx Lidotral - Clinical Pharmacology
Mechanism of Action: Lidotral™ 3.88% Cream releases lidocaine from a mild acidic vehicle to stabilize the neuronal membrane by inhibiting the ionic fluxes required for initiation and conduction of impulses, thereby effecting local anesthetic action. A mild acidic vehicle lowers pH to increase protection against alkaline irritations and to provide a favorable environment for healing.
Pharmacokinetics: Lidocaine may be absorbed following topical administration to mucous membranes, its rate and extent of absorption depending upon the specific site of application, duration of exposure, concentration, and total dosage. In general, the rate of absorption of local anesthetic agents following topical application occurs most rapidly after intratracheal administration. Lidocaine is also well-absorbed from the gastrointestinal tract, but little intact drug appears in the circulation because of biotransformation of the liver.
Lidocaine is metabolized rapidly by the liver, and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjungation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylid...



