DermacinRx ZRM
Generic name: lidocaine, dimethicone
Dosage form: kit
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Lidocaine Patch 5%
Rx Only
DESCRIPTION
Lidocaine patch 5% is comprised of an adhesive material containing 5% lidocaine, which is applied to a white non-woven polyethylene terephthalate (PET) material backing and covered with a transparent PET release liner. The release liner is removed prior to application to the skin. The size of the patch is 10 cm x 14 cm.
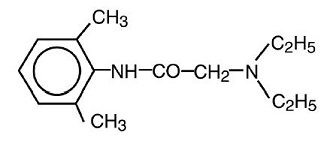
Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), has an octanol:water partition ratio of 43 at pH 7.4, and has the following structure:
Each adhesive patch contains 700 mg of lidocaine (50 mg per gram adhesive) in an aqueous base. It also contains the following inactive ingredients: glycerin, D-sorbitol, propylene glycol, polyvinyl alcohol, urea, sodium polyacrylate, carboxymethylcellulose sodium, gelatin, polyacrylic acid, kaolin, tartaric acid, dihydroxyaluminum aminoacetate, methylparaben, propylparaben, and edetate disodium.
CLINICAL PHARMACOLOGY
PharmacodynamicsLidocaine is an amide-type local anesthetic agent and is suggested to stabilize neuronal membranes by inhibiting the ionic fluxes required for the initiation and conduction of impulses.
The penetration of lidocaine into intact skin after application of lidocaine patch is sufficient to produce an analgesic effect, but less than the amount necessary to produce a complete sensory block.
PharmacokineticsAbsorption: The amount of lidocaine systemically absorbed from lidocaine patch is directly related to both the duration of application and the surface area over which it ..



