Dextrose and Sodium Chloride Injection
Dosage form: injection, solution
Medically reviewed by Drugs.com. Last updated on Jan 1, 2021.
On This Page
| 5% Dextrose and 0.9% Sodium Chloride Injection, USP |
Rx only
DESCRIPTION
Dextrose and Sodium Chloride Injection, USP solutions are sterile and nonpyrogenic. They are large volume parenteral solutions containing 5 grams per 100 mL of Dextrose monohydrate and 0.9 grams per 100 mL of Sodium Chloride in water for injection intended for intravenous administration. Electrolytes per 1000 mL: sodium (Na+), 154 mEq; chloride (Cl-) 154 mEq. The osmolarity is 560 mOsmol/L (calc.), which is hypertonic. The caloric value is 170 kcal/L. The pH is 4.3 (3.5 to 6.5).
The solutions contain no bacteriostat, antimicrobial agent or added buffer and each is intended only as a single-dose injection. When smaller doses are required the unused portion should be discarded.
The solutions are parenteral fluid, nutrient and electrolyte replenishers.
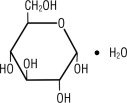
Dextrose, USP is chemically designated D-glucose monohydrate (C6H12O6 • H2O), a hexose sugar freely soluble in water. It has the following structural formula:
Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder freely soluble in water.
Water for Injection, USP is chemically designated H2O.
The flexible container is fabricated from a specially formulated non-plasticized, film containing polypropylene and thermoplastic elastomers (freeflex® bag). The amount of water that can permeate from the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the flexible container can leach out certain of the container's chemical components in very small amounts within the expiration period. The suitabili...