Dextrose in Ringer's
Generic name: dextrose, sodium chloride, calcium chloride, and potassium chloride
Dosage form: injection
Drug class:Intravenous nutritional products
Medically reviewed by Drugs.com. Last updated on Jan 24, 2022.
On This Page
Dextrose in Ringer's Description
Each 100 mL of 5% Dextrose in Ringer's Injection contains: Hydrous Dextrose USP 5 g; Sodium Chloride USP 0.86 g; Calcium Chloride Dihydrate USP 0.033 g; Potassium Chloride USP 0.03 g; Water for Injection USP qs
pH: 4.3 (3.5–6.5); Calories per liter: 170
Calculated Osmolarity: 560 mOsmol/liter, hypertonic
Concentration of Electrolytes (mEq/liter): Sodium 147 Potassium 4
Calcium 4.5 Chloride 156
5% Dextrose in Ringer's Injection is sterile, nonpyrogenic, and contains no bacteriostatic or antimicrobial agents.
The formulas of the active ingredients are:
| Ingredients | Molecular Formula | Molecular Weight |
|---|---|---|
| Sodium Chloride USP | NaCl | 58.44 |
| Potassium Chloride USP | KCl | 74.55 |
| Calcium Chloride Dihydrate USP | CaCl2•2H2O | 147.02 |
| Hydrous Dextrose USP | 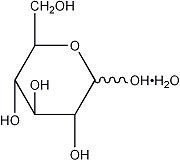 | 198.17 |
The EXCEL® Container is Latex-free; PVC-free; and DEHP-free.
The plastic container is made from a multilayered film specifically developed for parenteral drugs. It contains no plasticizers and exhibits virtually no leachables. The solution contact layer is a rubberized copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during administration. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrie..



