Dextrose Injection 70%
Dosage form: injection, solution
Drug class:Glucose elevating agents
Medically reviewed by Drugs.com. Last updated on Jun 21, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Package Insert
Administer only after dilution via central venous catheter.
Dextrose Injection 70% Description
Each 100 mL of 70% Dextrose Injection, USP contains:
Hydrous Dextrose USP 70 g; Water for Injection USP qs
pH: 4.6 (3.2-6.5); Calculated Osmolarity: 3530 mOsmol/liter
Calories per liter: 2380; Calculated Specific Gravity 1.23
70% Dextrose Injection, USP is sterile, nonpyrogenic and contains no bacteriostatic or antimicrobial agents.
The formula of the active ingredient is:
| Ingredient | Molecular Formula | Molecular Weight |
|---|---|---|
| Hydrous Dextrose USP | 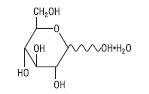 | 198.17 |
Dextrose Injection 70% - Clinical Pharmacology
70% Dextrose Injection, USP provides calories and is a source of water for hydration. This solution is capable of inducing diuresis depending on the clinical condition of the patient.
Dextrose is readily metabolized, may decrease losses of body protein and nitrogen, promotes glycogen deposition and decreases or prevents ketosis if sufficient doses are provided.
70% dextrose solution provides a maximum source of calories in a minimal fluid volume.



