Alcohol and Dextrose
Generic name: Alcohol and Dextrose monohydrate
Dosage form: injection, USP
Drug class:Miscellaneous uncategorized agents
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
On This Page
Alcohol and Dextrose Description
Each 100 mL of 5% Alcohol and 5% Dextrose Injection USP contains:
Alcohol USP 5 mL; Hydrous Dextrose USP 5 g
Water for Injection USP qs
pH: 5.0 (3.5–6.5); Calories per liter: 450
Calculated Osmolarity: 1125 mOsmol/liter, hypertonic
Each 100 mL of 10% Alcohol and 5% Dextrose Injection USP contains: Alcohol USP 10 mL; Hydrous Dextrose USP 5 g
Water for Injection USP qs
pH: 4.6 (3.5–6.5); Calories per liter: 720
Calculated Osmolarity: 1995 mOsmol/liter, strongly hypertonic
These intravenous solutions are sterile, nonpyrogenic, hypertonic and contain no bacteriostatic or antimicrobial agents.
The formulas of the active ingredients are:
| Ingredients | Molecular Formula | Molecular Weight |
|---|---|---|
| Alcohol USP | CH3CH2OH | 46.07 |
| Hydrous Dextrose USP | 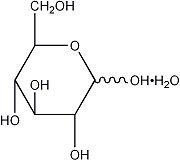 | 198.17 |
Alcohol and Dextrose - Clinical Pharmacology
Alcohol and Dextrose Injections USP are an intravenous source of calories. In the average adult, pure ethyl alcohol is metabolized at a rate of 10 to 20 mL per hour. Sedative effects of alcohol occur if the rate of infusion exceeds the rate of metabolism. Dextrose (D-glucose) can be infused at a maximum rate of approximately 0.5 to 0.85 g/kg of body weight/hr without producing significant glycosuria. Thus, the maximum rate that alcohol can be infused without producing sedative effects is



