Equioxx
Generic name: firocoxib paste
Dosage form: FOR ANIMAL USE ONLY
On This Page
Non-steroidal anti-inflammatory drug for oral use in horses only.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
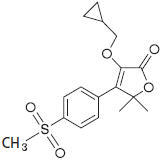
Equioxx® (firocoxib) belongs to the coxib class of non-narcotic, non-steroidal anti-inflammatory drugs. Firocoxib is a white crystalline compound described chemically as 3-(cyclopropylmethoxy)-4-(4-(methylsulfonyl)phenyl)-5,5-dimethylfuranone. The empirical formula is C17H20O5S, and the molecular weight is 336.4. The structural formula is shown below:
Indications
Equioxx Oral Paste is administered for up to 14 days for the control of pain and inflammation associated with osteoarthritis in horses.
Dosage and Administration
Always provide the Client Information Sheet with the prescription. The recommended dosage of Equioxx (firocoxib) for oral administration in horses is 0.045 mg/lb (0.1 mg/kg) of body weight once daily for up to14 days. In target animal safety studies, toxicity was seen at the recommended dose when the duration of treatment exceeded 30 days.
Each marking on the syringe will treat 250 pounds of body weight, and each notch corresponds to approximately a 50 lb weight increment. To deliver the correct dose, round the horse's body weight up to the nearest 50 pound increment (if the body weight is an exact 50 pound increment, do not round up). Unlock the knurled ring on the syringe plunger by rotating it ¼ turn. Slide the knurled ring along the plunger shaft so that the side nearest the barrel is at the appropriate 50 lb weight notch. Rotate the plunger ring ¼ turn to lock it in place and ensure it is locked.
Equioxx may be given with or without food.
Contraindications
Horses with hypersensitivity to firocoxib or other NSAIDs should not receive Equioxx Oral Paste.
Warnings
For oral use in horses only. Do not use in horses intended for human consumption.
Human Warnings
Not for use in humans. Keep this and all medications out of the reach of children. Consult a physician in case of accidental ingestion by humans.
Animal Safety
Clients should be advised to observe for signs of potential drug toxicity and be given a Client Information Sheet with each prescription.
For technical assistance or to report suspected adverse events, call 1-877-217-3543.
Precautions
Horses should undergo a thorough hist...



