Exall Liquid
Generic name:carbetapentane citrate and guaifenesin
Dosage form: liquid
Drug class:Upper respiratory combinations
Medically reviewed by Drugs.com. Last updated on Jul 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Exall Liquid
Rx OnlyAntitussive / Expectorant
DESCRIPTION: ExallTM Liquid is alcohol-free, dye-free, sugar-free, colorless liquid for oral administration having a cherry odor and flavor.
Each teaspoonful (5 mL) for oral administration contains:
Carbetapentane Citrate..... 10 mg
Guaifenesin.....100 mg
Inactive ingredients: Cherry Flavor, Citric Acid, Glycerin, Propylene Glycol, Purified Water, Sodium Citrate, Sodium Saccharin, Sorbitol.
Carbetapentane Citrate (1-Phenylcyclopentanecarboxylic acid 2-(2-diethylaminoethoxy) ethyl ester citrate) is a white crystalline powder.
It is freely soluble in water and chloroform. Its structure is as follows:
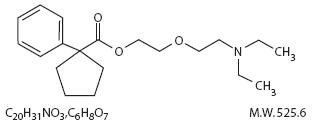
Guaifenesin (1,2-Propanediol, 3-(2-methoxyphenoxy)-, (±)-) is a white to slightly gray, crystalline powder, having a bitter taste.
It may have a slight characteristic odor. It is soluble in water, alcohol, chloroform, glycerin, and propylene glycol. Its structure is as follows:
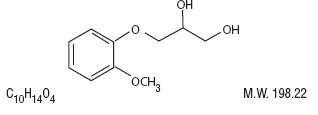
CLINICAL PHARMACOLOGY:
Antitussive and expectorant actions.
Carbetapentane Citrate is a centrally acting non-narcotic antitussive. Carbetapentane citrate has atropine-like and local anesthetic actions,
as well as temporarily controls and suppresses the cough reflex by selective depression of the medullary cough center. It has no significant
analgesic or sedative properties, does not depress respiration or predispose to addiction with usual doses.
Guaifenesin has an expectorant action, which increases the output of respiratory tract fluid by reducing adhesiveness and surface tension.
By increasing respirat



