Fibryga
Generic name:fibrinogen (human)
Dosage form: injection
Drug class:Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Dec 1, 2020.
On This Page
Indications and Usage for Fibryga
Fibryga is a human fibrinogen concentrate indicated for the treatment of acute bleeding episodes in adults and children with congenital fibrinogen deficiency, including afibrinogenemia and hypofibrinogenemia.
Fibryga is not indicated for dysfibrinogenemia.
Fibryga Dosage and Administration
For intravenous use only after reconstitution.
Dosage
Fibryga dosing, duration of dosing, and frequency of administration should be individualized based on the extent of bleeding, laboratory values, and the clinical condition of the patient.
The recommended target fibrinogen plasma level is 100 mg/dL for minor bleeding and 150 mg/dL for major bleeding.
Fibryga dose when baseline fibrinogen level is known
Dose should be individually calculated for each patient based on the target plasma fibrinogen level for the type of bleeding, actual measured plasma fibrinogen level and body weight , using the following age-specific formulas (see Pharmacokinetics [ 12.3 ] ):
Adults and adolescents 12 years of age and above:

Children 0 to <12 years of age:
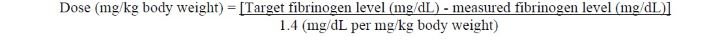
Fibryga dose when baseline fibrinogen level is not known
If the patient’s fibrinogen level is not known, the recommended dose is 70 mg/kg of body weight administered intravenously.
Monitor the patient’s fibrinogen level during treatment with Fibryga.
Additional infusions of Fibryga should be administered if the plasma fibrinogen level is below the accepted lower limit (80 mg/dL for minor bleeding, 130 mg/dL for major bleeding) of the target level until...



