Flagyl 375 Capsules
Generic name:metronidazole
Dosage form: capsule
Drug classes:Amebicides, Miscellaneous antibiotics
Medically reviewed by Drugs.com. Last updated on Dec 1, 2021.
On This Page
To reduce the development of drug-resistant bacteria and maintain the effectiveness of FLAGYL® and other antibacterial drugs, FLAGYL should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Metronidazole has been shown to be carcinogenic in mice and rats (See PRECAUTIONS). Unnecessary use of the drug should be avoided. Its use should be reserved for the conditions described in the INDICATIONS AND USAGE section below.
Flagyl 375 Capsules Description
FLAGYL (metronidazole) capsules, 375 mg is an oral formulation of the synthetic nitroimidazole antimicrobial agent, 2-methyl-5-nitro-1H-imidazole-1-ethanol, which has the following structural formula:
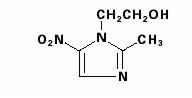
FLAGYL (metronidazole) capsules, 375 mg, (indicated below as Flagyl 375 Capsules) contain 375 mg of metronidazole USP. Inactive ingredients include corn starch, magnesium stearate, gelatin, black iron oxide, titanium dioxide, FD&C Green No. 3, and D&C Yellow No. 10.
Flagyl 375 Capsules - Clinical Pharmacology
Absorption
Disposition of metronidazole in the body is similar for both oral and intravenous dosage forms.
Flagyl 375 Capsules have been shown to have a rate and extent of absorption similar to metronidazole tablets (FLAGYL) and were bioequivalent at an equal single dose of 750 mg. In a study conducted with 23 adult, healthy, female volunteers, oral administration of two 375 mg FLAGYL capsules under fasted conditions produced a mean (±1 SD) peak plasma concentration (Cmax) of 21.4 (±2.8) mcg/mL with a mean Tmax of 1.6 (± 0.7) hours and a mean area under the plasma concentration-...



