Fluocinolone Ear Drops
Generic name: fluocinolone acetonide
Dosage form: otic oil
Drug class:Otic steroids
Medically reviewed by Drugs.com. Last updated on Apr 1, 2021.
On This Page
For Otic Use Only
Not for Ophthalmic Use
Fluocinolone Ear Drops Description
Fluocinolone acetonide oil, 0.01% (ear drops) contain fluocinolone acetonide {(6α, 11β, 16α)-6,9- difluoro-11,21-dihydroxy-16,17[(1-methylethylidene)bis(oxy)]-pregna-1,4-diene-3,20-dione, cyclic 16,17 acetal with acetone}, a synthetic corticosteroid. This formulation is also marketed as fluocinolone acetonide topical oil, 0.01% (body oil) for the treatment of atopic dermatitis and fluocinolone acetonide topical oil, 0.01% (scalp oil) for the treatment of psoriasis of scalp. Chemically, fluocinolone acetonide is C24H30F2O6. It has the following structural formula: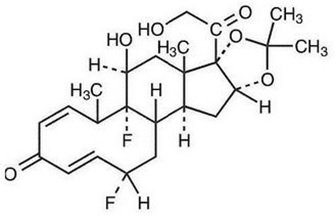
Fluocinolone acetonide has a molecular weight of 452.50. It is a white crystalline powder that is odorless, stable in light, and melts at 270°C with decomposition; soluble in alcohol, acetone and methanol; slightly soluble in chloroform; insoluble in water.
Each gram of fluocinolone acetonide oil, 0.01% (ear drops) contains approximately 0.11 mg of fluocinolone acetonide in a blend of oils, which contains isopropyl alcohol, isopropyl myristate, light mineral oil, oleth-2 and refined peanut oil NF.
Fluocinolone Ear Drops - Clinical Pharmacology
Like other topical corticosteroids, fluocinolone acetonide has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
Pharmacokinetics: The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle and the integrity of the epidermal barrier. Occlusion of topical corticos..



