Gadopentetate Injection
Generic name: gadopentetate dimeglumine
Dosage form: injection
Drug class:Magnetic resonance imaging contrast media
Medically reviewed by Drugs.com. Last updated on Jan 24, 2022.
On This Page
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with
impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic
information is essential and not available with non-contrasted MRI or other modalities. NSF may
result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs.
• Do not administer gadopentetate dimeglumine to patients with:
◦ chronic, severe kidney disease (GFR < 30 mL/min/1.73m2), or
◦ acute kidney injury (see CONTRAINDICATIONS).
• Screen patients for acute kidney injury and other conditions that may reduce renal function.
For patients at risk for chronically reduced renal function (for example, age >60 years,
hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory
testing.
Do not exceed the recommended gadopentetate dimeglumine dose and allow a sufficient period
of time for elimination of the drug from the body prior to any re-administration (see WARNINGS
AND PRECAUTIONS).
Gadopentetate Injection Description
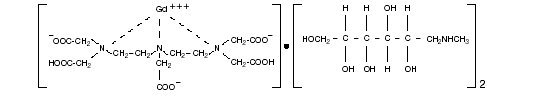
Gadopentetate dimeglumine Injection is the N-methylglucamine salt of the gadolinium complex of diethylenetriamine pentaacetic acid, and is an injectable contrast medium for magnetic resonance imaging (MRI). Gadopentetate dimeglumine Injection is provided as a sterile, clear, colorless to slightly yellow aqueous solution for intravenous injection.
Gadopentetate dimeglumine Injection is a 0.5-mol/L solution of 1-deoxy-1-(methylamino)-D-glucitol dihydrogen [N,N-bis[2-[bis(carboxymethyl)amino]ethyl] glycinato(5-)]gadolinate(2-)(2:1) with a molecular weight of 938, an empirical formula of C28H54GdN5O20, and has the following structural formula:
Each mL contains 469.0...