Hailey FE 1.5/30
Generic name:norethindrone acetate and ethinyl estradiol and ferrous fumarate
Dosage form: tabkets
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on Jun 1, 2022.
On This Page
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Hailey FE 1.5/30 Description
Hailey® Fe 1.5/30 (norethindrone acetate and ethinyl estradiol tablets, USP and ferrous fumarate tablets, USP) is a progestogen-estrogen combination.
Hailey FE 1.5/30 (norethindrone acetate and ethinyl estradiol tablets, USP and ferrous fumarate tablets, USP): provides a continuous dosage regimen consisting of 21 oral contraceptive tablets and seven ferrous fumarate tablets. The ferrous fumarate tablets are present to facilitate ease of drug administration via a 28-day regimen, are non-hormonal, and do not serve any therapeutic purpose.
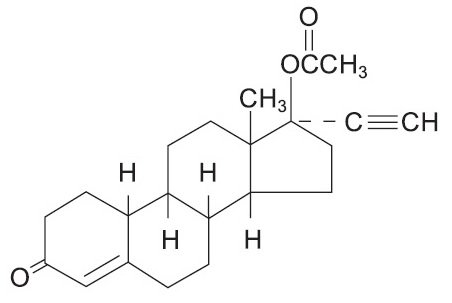
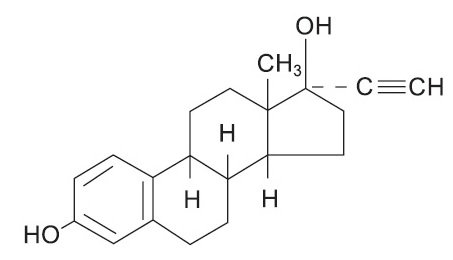
Each light green to green tablet, for oral administration, contains 1.5 mg norethindrone acetate, USP [(17α)-17-(acetyloxy)-19-norpregn-4-en-20-yn-3-one] and 30 mcg ethinyl estradiol, USP [19-Nor-17α-pregna-1,3,5(10)-trien-20-yne-3,17-diol]. The tablets also contain the following inactive ingredients: acacia, corn starch, D&C Yellow #10, FD&C Blue #1, FD&C Yellow #6, lactose monohydrate, magnesium stearate, sucrose, and talc.
The structural formulas are as follows:
Each brown to dark brown tablet for oral administration contains ferrous fumarate, acacia, corn starch, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, sucralose, and talc. The ferrous fumarate tablets do not serve any therapeutic purpose.
Hailey FE 1.5/30 - Clinical Pharmacology
C...