Hexabrix
Generic name:ioxaglate meglumine and ioxaglate sodium
Dosage form: injection
Drug class:Ionic iodinated contrast media
Medically reviewed by Drugs.com. Last updated on Feb 21, 2022.
On This Page
Hexabrix Description
Hexabrix is a sterile, non-pyrogenic, aqueous solution intended for use as a diagnostic radiopaque medium. Hexabrix contains 39.3% w/v N-(2-hydroxyethyl)-2,4,6-triiodo-5-[2-[2,4,6-triiodo-3-(N-methylacetamido)-5-(methylcarbamoyl) benzamido] acetamido]-isophthalamic acid, compounded with 1-deoxy-1-(methylamino)-D-glucitol (1:1) and 19.6% w/v sodium N-(2-hydroxyethyl)-2,4,6 triiodo-5-[2-[2,4,6-triiodo-3-(N-methylacetamido)-5-(methylcarbamoyl) benzamido] acetamido]-isophthalamate.
The two salts have the following structural formulae:
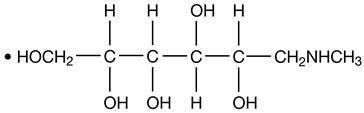
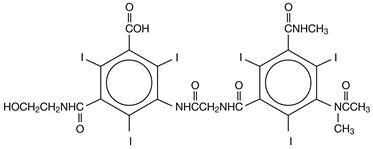
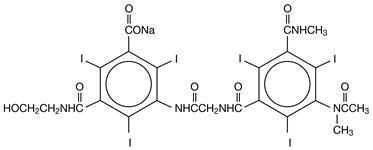
†Licensed by Guerbet, S.A.
Registered U.S. Patent and Trademark Office
Each milliliter contains 393 mg of ioxaglate meglumine, 196 mg of ioxaglate sodium and 0.10 mg edetate calcium disodium as a stabilizer. The solution contains 3.48 mg (0.15 mEq) sodium in each milliliter and provides 32% (320 mg/mL) organically bound iodine.
Solutions of ioxaglate (Hexabrix) provide six iodine atoms for each two dissociated ions. Hexabrix is an ionic contrast agent. Hexabrix has an osmolarity of approximately 460 mOsmol/L, an osmolality of approximately 600 mOsmol/kg of water and is, therefore, hypertonic under conditions of use.
Hexabrix has a viscosity (cps) of 15.7 at 20°C and 7.5 at 37°C. The pH has been adjusted to 6.0 to 7.6 with meglumine, sodium hydroxide or ioxaglic acid.
Hexabrix is a clear, colorless to pale yellow solution containing no undissolved solids. Crystallization does not occur at normal room temperatures. It is supplied in containers from which the air has been displaced by nitrogen.
Hexabrix - Clinical Pharmacology
Intravascular injection of a radiopaque diag...



