HMS
Generic name: medrysone
Dosage form: Ophthalmic Suspension 1%
Drug class:Ophthalmic steroids
Medically reviewed by Drugs.com. Last updated on Jan 24, 2022.
On This Page
Sterile
HMS Description
HMS® (medrysone ophthalmic suspension) 1% is a topical anti-inflammatory agent for ophthalmic use.
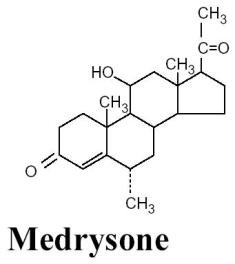
Chemical Name:
11β-hydroxy-6α-methylpregn-4-ene-3,20-dione
Structural Formula:
Contains: Active: Medrysone 1%. Preservative: benzalkonium chloride 0.004%.
Inactives: edetate disodium; hypromellose; polyvinyl alcohol 1.4%; potassium chloride; purified water; sodium chloride; sodium phosphate, dibasic; sodium phosphate, monobasic; and sodium hydroxide to adjust the pH (6.2 - 7.5).
HMS - Clinical Pharmacology
HMS® (medrysone ophthalmic suspension) is a synthetic corticosteroid with topical anti-inflammatory activity. Glucocorticoids inhibit the edema, fibrin deposition, capillary dilation and phagocytic migration of the acute inflammatory response as well as capillary proliferation, deposition of collagen, and scar formation. HMS® (medrysone ophthalmic suspension) has less anti-inflammatory potency than 0.1% dexamethasone.
Indications and Usage for HMS
HMS® (medrysone ophthalmic suspension) is indicated for the treatment of allergic conjunctivitis, vernal conjunctivitis, episcleritis, and epinephrine sensitivity.
Contraindications
HMS® suspension is contraindicated in most viral diseases of the cornea and conjunctiva, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures. HMS® suspension is also contraindicated in individuals with known or suspected hypersensitivity to any of the ingredients of this preparation and to other corticosteroids.
Warnings
HMS