Homatropine Ophthalmic Solution
Generic name: homatropine hydrobromide
Dosage form: ophthalmic solution
Drug class:Mydriatics
Medically reviewed by Drugs.com. Last updated on Feb 21, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
DESCRIPTION SECTION
Homatropine hydrobromide is an anticholinergic prepared as a sterile topical ophthalmic solution. Chemical Name: Benzeneacetic acid, a-hydroxy-,8-methyl-8-azabicyclo [3.2.1]-oct-3-yl ester, hydrobromide, endo-(±)-.
The active ingredient is represented by the chemical structure,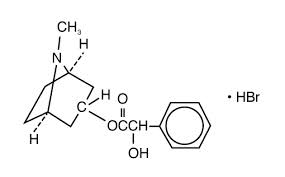
Each mL containes: Active: Homatropine Hydrobromide 5.0%. Preservative: Benzalkonium Chloride 0.005%.
Inactive: Boric Acid, Edetate Disodium, Potassium Chloride, Water for Injection. Boric Acid or Sodium Carbonate may be added to adjust the pH.
INDICATIONS & USAGE SECTION
A moderately long-acting mydriatic and cycloplegic for cycloplegic refraction and in the treatment of inflammatory conditions of the uveal tract. For pre and postoperative states when mydriasis is required. Use as an optical aid in some
cases of axial lens opacities.
CLINICAL PHARMACOLOGY SECTION
This anticholinergic preparation blocks the responses of the sphincter muscle of the iris and the accommodative muscle of the ciliary body to cholinergic stimulation, producing pupillary dilation (mydriasis) and paralysis of accommodation (cycloplegia).
CONTRAINDICATIONS SECTION
Contraindicated in persons with primary glaucoma or a tendency toward glaucoma, e.g. narrow anterior chamber angle, and in those persons showing hypersensitivity to any component of this preparation.
PREGNANCY SECTION
Pregnancy Category C. Animal reproduction studies have not been conducted with homatropine hydrobromine. It is



