HT-Tuss DM Elixir
Generic name:dextromethorphan hydrobromide and guaifenesin
Dosage form: elixir
Drug class:Upper respiratory combinations
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
HT-Tuss DM Elixir Description
Each 5 mL (one teaspoonful) of HT-Tuss DM Elixir contains:
Dextromethorphan hydrobromide . . . 20 mg
Guaifenesin . . . . 200 mg
Alcohol . . . . . . . . . . . 5%
Also contains: Citric acid, FD&C blue #1, FD&C red #3, FD&C red #40, high fructose corn syrup, natural & artificial grape flavor, propylene glycol, purified water, saccharin sodium, sodium benzoate. Sodium citrate may be used to adjust pH.
Dextromethorphan hydrobromide, a synthetic monopioid antitussive, is a salt of the methyl ether of the dextrorotatory isomer of levorphanol, an opioid analgesic. The chemical name is 3- methoxy-17-methyl-9α, 13α, 14α-morphinan hydrobromide monohydrate. It has the following structural formula:
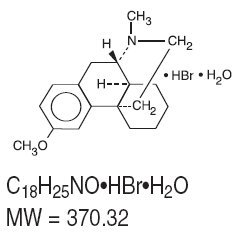
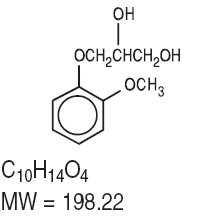
Guaifenesin is an expectorant. The chemical name is (±)-3-(o- Methoxyphenoxy)-1,2- propanediol. It has the following structural formula:
HT-Tuss DM Elixir - Clinical Pharmacology
HT-Tuss DM Elixir combines the cough suppressant dextromethorphan hydrobromide and the expectorant guaifenesin.
Dextromethorphan hydrobromide is a non-opioid antitussive agent. Dextromethorphan temporarily controls and suppresses the cough reflex by a direct action on the cough center. It has no significant analgesic or sedative properties, does not depress respiration or predispose to addiction with usual doses. In therapeutic dosage, dext...



