Hypaque
Generic name:diatrizoate sodium
Dosage form: Oral Powder
Drug class:Ionic iodinated contrast media
Medically reviewed by Drugs.com. Last updated on Jan 24, 2022.
For Examination of the Gastrointestinal Tract
| NOT FOR INTRATHECAL USE |
RX ONLY
On This Page
Hypaque Description
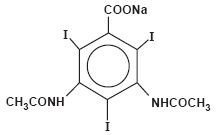
Hypaque Sodium (Diatrizoate Sodium, USP) is sodium 3,5-diacetamido-2, 4, 6-triiodobenzoate (C11H8I3N2NaO4) and contains 59.87 percent iodine. It is available as a powder. The powder (for preparing radiopaque solutions) provides about 600 mg organically bound iodine per gram of powder and contains caramel as a coloring agent. The structural formula is as follows:
Inactive Ingredients: Caramel, Polysorbate 80.
Hypaque - Clinical Pharmacology
When administered orally or given as an enema, the medium produces excellent opacification and delineation of the upper and lower gastrointestinal tract; however, because of dilution, contrast in the small bowel may be unsatisfactory. Hypaque solutions are particularly valuable when a more viscous agent, such as barium sulfate which is not water soluble, is unsuitable or potentially harmful.
From 0.04 to 1.2 percent of the medium may be absorbed from the gastrointestinal tract after oral administration. In some patients, particularly in infants and patients with engorgement of the intestinal mucosa, sufficient absorption to cause some visualization of the urinary tract may occur occasionally. Hypaque is also absorbed across the peritoneum and pleura.
Indications and Usage for Hypaque
This medium is indicated for radiographic examination of the gastrointestinal tract following oral or rectal administration.
Warnings
SEVERE ADVERSE EVENTS-INADVERTENT INTRATHECAL ADMINISTRATION
Severe adverse reactions have been reported due to the inadvertent intrathecal administration of iodinated contrast media that are not indicated for intrathecal use. These serious adverse reactions include: death, convulsions, cerebral hemorrha...



