Hypaque Injection
Generic name: diatrizoate sodium
Dosage form: Injection, USP 50%
Drug class:Ionic iodinated contrast media
Medically reviewed by Drugs.com. Last updated on Mar 22, 2022.
Sterile Aqueous Injection
For Excretory Urography
Cerebral Angiography
Peripheral Angiography
Aortography
Intraosseous Venography
Direct Cholangiography
Hysterosalpingography
Splenoportography
| NOT FOR INTRATHECAL USE |
Rx ONLY
On This Page
Hypaque Injection Description
HYPAQUE sodium, brand of diatrizoate sodium, is a radiopaque diagnostic agent, water-soluble organic iodide contrast medium. In pure form, it contains 59.87 percent organically bound iodine.
The 50 percent (w/v) solution contains 300 mg iodine per mL and 0.8 mEq (18.1 mg) sodium per mL. It has an osmolality of 1515 mosm/kg (determined by VPO), and is hypertonic to blood. As a point of information only, a 10 percent solution (w/v) is isotonic. The viscosity (cp) is about 3.25 at 25° C and 2.34 at 37° C. Sodium carbonate and hydrochloric acid have been added to adjust pH between 6.5 and 7.7.The pKa is 3.4 for diatrizoic acid. If a solution of this medium is chilled, crystals may form but readily dissolve if the vial is placed in moderately hot water before use; cool to body temperature before injecting.
The sterile aqueous solution is clear and nearly colorless. It is relatively thermostable and may be autoclaved without harmful effects, although it should be protected from strong light. The 50 percent solution contains edetate calcium disodium 1:10,000 as a sequestering stabilizing agent.
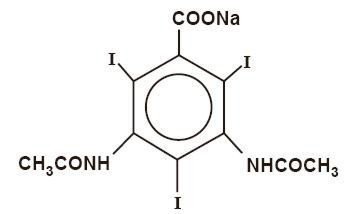
Diatrizoate sodium is a triiodinated benzoic acid derivative, the sodium salt of 3,5-diacetamido-2,4,6-triiodobenzoate with a molecular weight of 635.90, and has the following structural formula:
Hypaque Injection - Clinical Pharmacology
Intravascular injection of a radiopaque diagnostic agent opacifies those vessels in the path of



