Hyperstat
Generic name:diazoxide
Dosage form: Injection
Drug classes:Agents for hypertensive emergencies, Glucose elevating agents
Medically reviewed by Drugs.com. Last updated on Apr 21, 2022.
For Intravenous Use in
Hospitalized Patients Only
PRODUCT INFORMATION
On This Page
The Hyperstat brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Hyperstat Description
Hyperstat I.V. Injection is a non-diuretic benzothiadiazine antihypertensive agent. Each ampule (20 mL) contains 300 mg diazoxide, USP, in a clear, sterile, colorless aqueous solution; the pH is adjusted to approximately 11.6 with sodium hydroxide.
Diazoxide has the following structural formula:
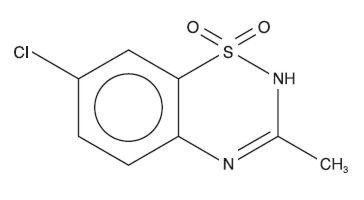
Diazoxide is 7-chloro-3-methyl-2H-1,2,4-benzothiadiazine 1,1-dioxide, with the empirical formula C8H7ClN2O2S, and the molecular weight 230.7. It is a white crystalline powder practically insoluble to sparingly soluble in water.
Hyperstat - Clinical Pharmacology
Hyperstat I.V. Injection produces a prompt reduction of blood pressure in man by relaxing smooth muscle in the peripheral arterioles. Cardiac output is increased as blood pressure is reduced. Studies in animals demonstrate that coronary blood flow is maintained, while renal blood flow is increased after an initial decrease.
Transient hyperglycemia occurs in the majority of patients treated with Hyperstat, but usually requires treatment only in patients with diabetes mellitus. It will respond to the usual management measures, including insulin.
Blood glucose levels should be monitored, especially in patients with diabetes and in those requiring multiple injections of diazoxide. Cataracts have been observed in a few animals receiving repeated daily doses of intravenous diazoxide.
Since diazoxide causes sodium retention, repeated injections may ..



