Hyronan
Generic name: sodium hyaluronate, lidocaine, isopropyl alcohol
Dosage form: kit
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
LIDOCAINE HYDROCHLORIDE INJECTION, USP
Preservative-Free
For Infiltration and Nerve Block
Rx only
Hyronan Description
Lidocaine Hydrochloride Injections are sterile, nonpyrogenic, aqueous, isotonic solutions that contain a local anesthetic agent and are administered parenterally by injection. See INDICATIONSAND USAGE for specific uses.
Each mL of the 2% solution contains lidocaine hydrochloride 20 mg and sodium chloride 6 mg. The pH of these solutions is adjusted to approximately 5.0 to 7.0 with sodium hydroxide and/or hydrochloric acid.
Lidocaine Hydrochloride Injection solutions contain lidocaine hydrochloride which is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, monohydrochloride and has the molecular wt. 270.8. Lidocaine HCl (C 14H 22N 2O • HCl) has the following structural formula:
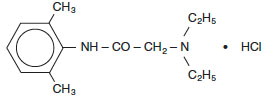
Hyronan - Clinical Pharmacology
Mechanism of Action
Lidocaine HCl stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses thereby effecting local anesthetic action.
Hemodynamics
Excessive blood levels may cause changes in cardiac output, total peripheral resistance, and mean arterial pressure. With central neural blockade these changes may be attributable to block of autonomic fibers, a direct depressant effect of the local anesthetic agent on various components of the cardiovascular system, and/or the beta-adrenergic receptor stimulating action of epinephrine when present. The net effect is normally a modest hypotension when the recommended dosages are n...



