Indigo Carmine
Generic name: indigotindisulfonate sodium
Dosage form: injection, solution
Drug class:Miscellaneous diagnostic dyes
Medically reviewed by Drugs.com. Last updated on Nov 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Rx Only
Indigo Carmine Description
Each mL contains: Indigotindisulfonate Sodium 8 mg, Water for Injection q.s. pH adjusted, when necessary, with Citric Acid and/or Sodium Citrate. Sterile, nonpyrogenic.
Sufficient Indigo Carmine is contained in each 5 mL ampule to permit accurate withdrawal and administration of the full dose. It gives a deep blue solution when dissolved in water.
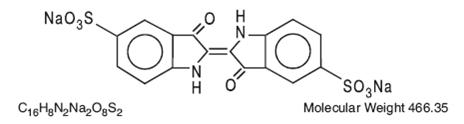
The structural formula is:
Indigo Carmine - Clinical Pharmacology
Indigo Carmine is excreted largely by the kidneys, retaining its blue color during passage through the body.
Elimination of the dye begins soon after injection, appearing in the urine within 10 minutes in average cases. The biological half-life is 4 to 5 minutes following intravenous injection. Larger quantities are necessary when intramuscular injection is employed. Appearance time and elimination are delayed following intramuscular injection.
Indications and Usage for Indigo Carmine
Originally employed as a kidney function test, the chief application of Indigo Carmine at present is localizing ureteral orifices during cystoscopy and ureteral catheterization.
Contraindications
Indigo Carmine is contraindicated in patients who have previously experienced an adverse reaction following its use.
Warnings
An occasional idiosyncratic drug re...



