Indium DTPA
Generic name: Pentetate Indium Disodium In 111
Dosage form: solution
Drug class:Diagnostic radiopharmaceuticals
Medically reviewed by Drugs.com. Last updated on Nov 22, 2021.
Rx ONLY
On This Page
Indium DTPA Description
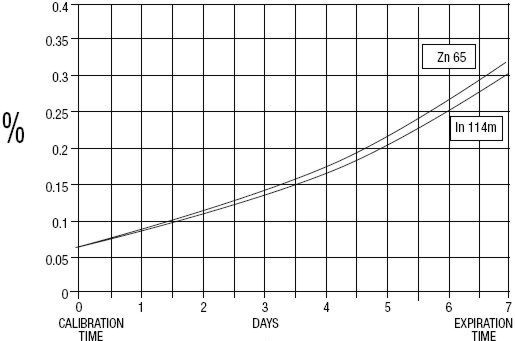
GE Healthcare (Medi-Physics, Inc.) Indium DTPA In 111 is a diagnostic drug for intrathecal use. It is available as a sterile, pyrogen-free, isotonic, aqueous solution, buffered to pH 7 to 8. At calibration time, each milliliter contains 37 MBq, 1 mCi of Pentetate Indium Disodium In 111 (no-carrier-added), 20 to 50 µg of pentetic acid, and sodium bicarbonate for pH adjustment. The drug is to be discarded after single use. Radionuclidic purity at calibration time is at least 99.88% with less than 0.06% Indium In 114m and 0.06% Zinc Zn 65. The concentration of each radionuclidic contaminant changes with time. Graph 1 shows maximum concentration of each radionuclidic impurity as a function of time.
Graph 1 - Radionuclidic Impurities
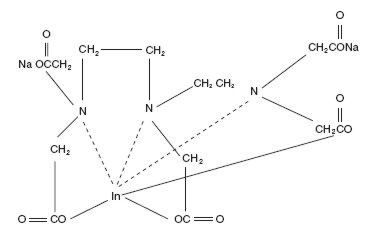
The chemical names are 1. Indate(2-)-111In-[N,N-bis[2-[bis-(carboxymethyl)amino]ethyl]glycinato(5-)]-disodium; and 2. Disodium [N,N-bis[2-(carboxymethyl)amino]glycinato(5-)]-indate (2-)111In.
Molecular formula: C14H18O10N3111In Na2
Molecular weight: 545.29
Structural formula:
PHYSICAL CHARACTERISTICS
Indium 111 decays by electron capture with a physical half-life of 67.9 hours.* The energies of the photons that are useful for detection and imaging studies are listed in Table 1.
| Radiation | <...
|---|



