Inflammacin
Generic name: diclofenac sodium, capsaicin
Dosage form: kit
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Diclofenac Sodium Delayed-Release Tablets USP
Rx only
Prescribing Information
On This Page
Cardiovascular Risk
Cardiovascular Thrombotic Events
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use [see WARNINGS and PRECAUTIONS].
- Diclofenac sodium delayed-release tablets are contraindicated in the setting of coronary artery bypass graft (CABG) surgery [see CONTRAINDICATIONS and WARNINGS].
Gastrointestinal Risk
- NSAIDs cause an increased risk of serious gastrointestinal adverse events including inflammation, bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events. [see WARNINGS].
DESCRIPTION
Diclofenac, as the sodium salt, is a benzene-acetic acid derivative. The chemical name is 2-[(2,6-dichlorophenyl)amino] benzeneacetic acid, monosodium salt. The molecular weight is 318.14. Its molecular formula is C 14H 10Cl 2NNaO 2, and it has the following structural formula:
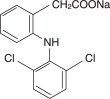
Each enteric-coated tablet for oral administration contains 75 mg of diclofenac sodium. In addition, each tablet contains the followin..



