Isolyte S
Generic name:sodium chloride, sodium gluconate, sodium acetate, potassium chloride, and magnesium chloride
Dosage form: injection, solution
Drug class:Intravenous nutritional products
Medically reviewed by Drugs.com. Last updated on Apr 21, 2022.
On This Page
Isolyte S Description
Each 100 mL of Isolyte® S (Multi-Electrolyte Injection) contains:
Sodium Chloride USP 0.53 g; Sodium Gluconate USP 0.5 g
Sodium Acetate Trihydrate USP 0.37 g; Potassium Chloride USP 0.037 g
Magnesium Chloride Hexahydrate USP 0.03 g
Water for Injection USP qs
pH adjusted with Glacial Acetic Acid USP
pH: 6.7 (6.3–7.3)
Calculated Osmolarity: 295 mOsmol/liter
Concentration of Electrolytes (mEq/liter): Sodium 140; Potassium 5
Magnesium 3; Chloride 98; Acetate (CH3COO−) 27
Gluconate (HOCH2(CHOH)4COO−) 23
Isolyte® S is sterile, nonpyrogenic, and contains no bacteriostatic or antimicrobial agents.
The formulas of the active ingredients are:
| Ingredients | Molecular Formula | Molecular Weight |
|---|---|---|
| Sodium Chloride USP | NaCl | 58.44 |
| Sodium Acetate Trihydrate USP | CH3COONa•3H2O | 136.08 |
| Potassium Chloride USP | KCl | 74.55 |
| Magnesium Chloride Hexahydrate USP | MgCl2•6H2O | 203.30 |
| Sodium Gluconate USP | 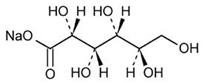 | 218.14 |
Not made with natural rubber latex, PVC or DEHP.
The plastic container is made from a multilayered film specifically developed for parenteral drugs. It contains no plasticizers and exhibits virtually no leachables. The solution contact layer is a rubberized copolymer of ethylen



