Kalosar
Generic name:calcipotriene
Dosage form: ointment
Drug class:Topical antipsoriatics
Medically reviewed by Drugs.com. Last updated on Apr 21, 2022.
On This Page
Rx Only
FOR TOPICAL DERMATOLOGIC USE ONLY.
Not for Ophthalmic, Oral or Intravaginal Use.
Kalosar Description
Kalosar™ (calcipotriene) Ointment USP, 0.005% contains the compound calcipotriene, USP, a synthetic vitamin D3 derivative for topical dermatological use.
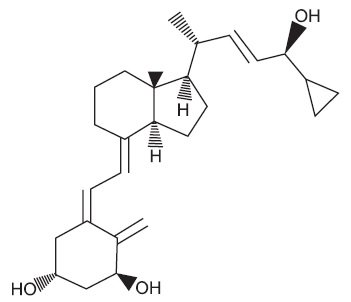
Chemically, calcipotriene, USP is 24-cyclopropyl-(1α,3β,5Z,7E,22E,24S)-9,10-Secochola-5,7,10(19),22-tetraene-1,3,24-trioI; with the empirical formula C27H40O3, a molecular weight of 412.62, and the following structural formula:
Calcipotriene, USP is a white or almost white, crystalline powder. Kalosar (calcipotriene) Ointment USP, 0.005% contains calcipotriene, USP 50 mcg/g in an ointment base of disodium phosphate dihydrate, edetate disodium, mineral oil, white petrolatum, propylene glycol, dl-alpha-tocopherol, steareth-2 and purified water.
Kalosar - Clinical Pharmacology
Clinical studies with radiolabelled calcipotriene ointment indicate that approximately 6% (± 3%, SD) of the applied dose of calcipotriene is absorbed systemically when the ointment is applied topically to psoriasis plaques or 5% (± 2.6%, SD) when applied to normal skin, and much of the absorbed active is converted to inactive metabolites within 24 hours of application.
Vitamin D and its metabolites are transported in the blood, bound to specific plasma proteins. The active form of the vitamin, 1, 25-dihydroxy vitamin D3 (calcitriol), is known to be recycled via the liver and excreted in the bile. Calcipotriene metabolism following systemic uptake is rapid, and occurs via a similar pathway to the natural hormone. The primary metabolites are much less potent than the parent compound.
There is evidence that mate...