Kelnor 1/50
Generic name:ethynodiol diacetate and ethinyl estradiol
Dosage form: tablets
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on Dec 1, 2021.
On This Page
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Kelnor 1/50 Description
Kelnor® 1/50 (28 Day Regimen) (ethynodiol diacetate and ethinyl estradiol tablets USP). Each pink tablet contains 1 mg of ethynodiol diacetate, USP and 50 mcg of ethinyl estradiol, USP and the inactive ingredients include anhydrous lactose, magnesium stearate, microcrystalline cellulose, polacrilin potassium, and povidone. In addition, the coloring agents are D&C Red No. 30 Aluminum Lake and D&C Yellow No. 10 Aluminum Lake.
Each white tablet in the Kelnor 1/50 package is a placebo containing no active ingredients, and the inactive ingredients include anhydrous lactose, magnesium stearate and microcrystalline cellulose.
The chemical name for ethynodiol diacetate, USP is 19-nor-17α-pregn-4-en-20-yne-3β, 17-diol diacetate, and for ethinyl estradiol, USP it is 19-nor-17α-pregna-1, 3, 5 (10)-trien-20-yne-3, 17-diol. The structural formulas are as follows:
Ethynodiol Diacetate, USP
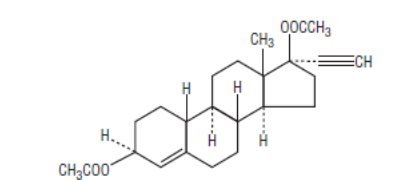
C24H32O4 M.W. 384.51
Ethinyl Estradiol, USP
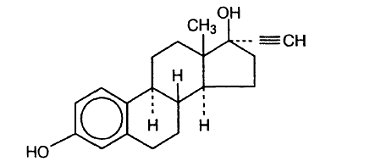
C20H24O2 M.W. 296.40
Therapeutic class: Oral contraceptive.
Kelnor 1/50 - Clinical Pharmacology
Combination oral contraceptives act primarily by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations in the genital tract, including changes in the cervical mucus ...



