Kemadrin
Generic name:procyclidine hydrochloride
Dosage form: tablets
Drug class:Anticholinergic antiparkinson agents
Medically reviewed by Drugs.com. Last updated on Jul 22, 2021.
5 mg Scored Tablets
On This Page
Description
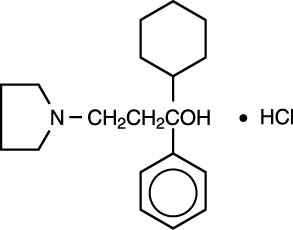
Kemadrin (procyclidine hydrochloride) is a synthetic antispasmodic compound of relatively low toxicity. It has been shown to be useful for the symptomatic treatment of parkinsonism (paralysis agitans) and extrapyramidal dysfunction caused by tranquilizer therapy. Procyclidine hydrochloride was developed at The Wellcome Research Laboratories as the most promising of a series of antiparkinsonism compounds produced by chemical modification of antihistamines. Procyclidine hydrochloride is a white crystalline substance which is soluble in water and almost tasteless. It is known chemically asα-cyclohexyl-α-phenyl-1-pyrrolidinepropanol hydrochloride and has the following structural formula:
Kemadrin is available in tablet form for oral administration. Each scored tablet contains5 mg procyclidine hydrochloride and the inactive ingredients corn and potato starch, lactose, and magnesium stearate.
Clinical Pharmacology
Pharmacologic tests have shown that procyclidine hydrochloride has an atropine-like action and exerts an antispasmodic effect on smooth muscle. It is a potent mydriatic and inhibits salivation. It has no sympathetic ganglion- blocking activity in doses as high as 4 mg/kg, as measured by the lack of inhibition of the response of the nictitating membrane to preganglionic electrical stimulation.
The intravenous LD50 in mice was about 60 mg/kg. Subcutaneously, doses of 300 mg/kg were not toxic. In dogs, the intraperitoneal administration of procyclidine hydrochloride in doses of 5 mg/kg caused maximal dilation of the pupil and inhibition of salivation, but had no toxic action. When the dose was increased to 20 mg/kg, the same symptoms occurred, and in addition there were tremors and ataxia lasting 4 to 5 hours. In one animal, convulsions occurred which were controlled by pentobarbital. In all animals behavior returned to normal within 24 hours.
Chronic toxicity tests in rats showed that the compound caused only a very slight retardation in growth, and no change in the erythrocyte count or the histological appearance of the lungs, liver, spleen, and kidney when as much as 10 mg/kg body weight was given subcutaneously daily for 9 weeks.
Indications
Kemadrin (pro...



