Kerafoam
Generic name:urea
Dosage form: aerosol, foam
Drug class:Topical emollients
Medically reviewed by Drugs.com. Last updated on Oct 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
The Kerafoam brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
DESCRIPTION:
Kerafoam® Emollient Foam is a keratolytic emollient foam which is a tissue softener for skin and/or nails. Kerafoam Emollient Foam contains 30% urea USP in an aqueous based emollient foam vehicle. Each gram of Kerafoam Emollient Foam contains 30% urea USP, ammonium lactate, cetyl alcohol NF, emulsifying wax NF, methylparaben NF, propylene glycol USP, propylparaben NF, purified water USP, steareth-10. Also contains: Propellant HFA-134a (1,1,1,2-tetrafluoroethane).
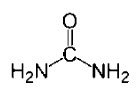
Urea USP is a diamide of carbonic acid with the following chemical structure:
Kerafoam - Clinical Pharmacology
Urea gently lyses/dissolves the intercellular matrix of surface skin cells loosening and allowing a shedding of rough, thickened and scaly hyperkeratotic skin. Urea also moisturizes and softens skin.
INDICATIONS
For softening, smoothing and removing rough scaling hyperkeratotic skin in conditions such as xerosis, ichthyosis, skin cracks and fissures, dermatitis, eczema, psoriasis, keratoses and calluses.
Contraindications
Known hypersensitivity to any of the listed ingredients. Discontinue use if hypersensitivity is observed.
Warnings
FOR EXTERNAL USE ONLY. Avoid contact with eyes, lips or mucous membranes.



