Generic name:fluocinonide
Dosage form: Topical Solution
Drug class:Topical steroids
Medically reviewed by Drugs.com. Last updated on May 23, 2022.
On This Page
The Lidex brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Description
Lidex solution 0.05% is intended for topical administration. The active component is the corticosteroid fluocinonide, which is the 21-acetate ester of fluocinolone acetonide and has the chemical name pregna-1,4-diene-3,20-dione,21-(acetyloxy)-6,9-difluoro-11-hydroxy-16,17-[(1-methylethylidene)bis(oxy)]-,(6α,11β,16α)-. It has the following chemical structure:
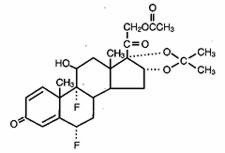
Lidex topical solution contains fluocinonide 0.5 mg/mL in a solution of alcohol (35%), citric acid, diisopropyl adipate, and propylene glycol. In this formulation, the active ingredient is totally in solution.
Clinical Pharmacology
Topical corticosteroids share anti-inflammatory, anti-pruritic and vasoconstrictive actions.
The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to ...



