Lidocaine and Hydrocortisone Gel
Generic name: ine hydrochloride, hydrocortisone acetate
Dosage form: rectal gel
Drug class:Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Feb 1, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
Rx Only
Anti-Inflammatory Anesthetic for Relief of Hemorrhoid Pain, Swelling and Inflammation
On This Page
DESCRIPTION:
Lidocaine 3% - Hydrocortisone 2.5% Gel Kit are indicated for the anti-inflammatory and anesthetic relief of itching, pain, soreness, and discomfort due to hemorrhoids, anal fissures, pruritus ani and similar conditions of the anal area.
ACTIVE INGREDIENTS:
Lidocaine 3% - Hydrocortisone 2.5% Gel Kit
lidocaine hydrochloride 3% (30mg) and hydrocortisone acetate 2.5% (25 mg) per gram.
INACTIVE INGREDIENTS:
aluminum sulfate, calcium acetate, cetyl alcohol, citric acid, glyceryl stearate (and) PEG-100 stearate, methylparaben, mineral oil, PEG-150 distearate, petrolatum, polycarbophil, propylene glycol, propylparaben, purified water, sodium citrate, sodium hydroxide, stearyl alcohol, xanthan gum.
CLINICAL PHARMACOLOGY:
MECHANISM OF ACTION:
Product releases lidocaine to stabilize the neuronal membrane by inhibiting the ionic fluxes required for initiation and conduction of impulses, thereby effecting local anesthetic action. Hydrocortisone acetate provides relief of inflammatory and pruritic manifestations of corticosteroid responsive dermatoses. Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), and has the following structure:
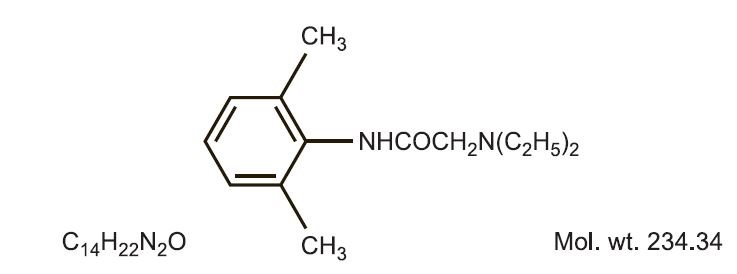
Hydrocortisone acetate has a chemical name pregn-4-ene-3, 20-dione, 21-(acetyloxy)-11,17- dihydroxy-(11ß)-. It has the following structural formula:
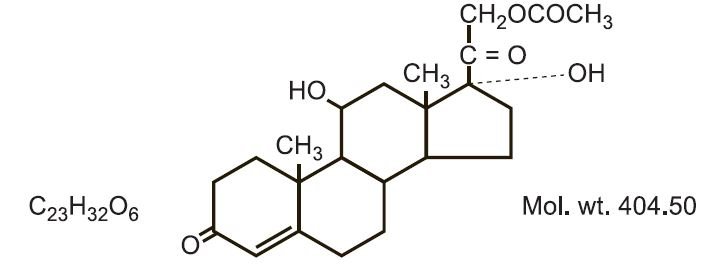
CONTRAINDICATIONS:
Product should not be used in patients with a history of sensitivity to any of its ingredients or adverse reactions to lidocaine or amide anesthetics, which usually do not cross-react with “caine” ester type anesthetics. If excessive irritation and significa...



