Mentadent Toothpolish
Generic name: sodium monofluorophosphate
Dosage form: gel, dentifrice
Drug class:Mouth and throat products
Medically reviewed by Drugs.com. Last updated on Oct 22, 2021.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Active ingredients
Sodium monofluorophosphate (0.84%)
Purpose
Anticavity toothpolish
Use aids in the prevention of dental decay
Warnings
Keep out of reach of children under 6 years of age.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- do not swallow
- adults and children 2 years and older: brush teeth thoroughly after meals or at least twice a day, or use as directed by a dentist or doctor
- instruct children under 6 years in good brushing and rinsing habits (to minimize swallowing)
- supervise children as necessary until capable of using without supervision
- children under 2 years ask a dentist or physician
Questions or comments? Call 1-800-786-5135 Monday-Friday 9am-5pm ET
Principal Display Panel
MENTADENT
Whitening Toothpolish
(85g)
Label image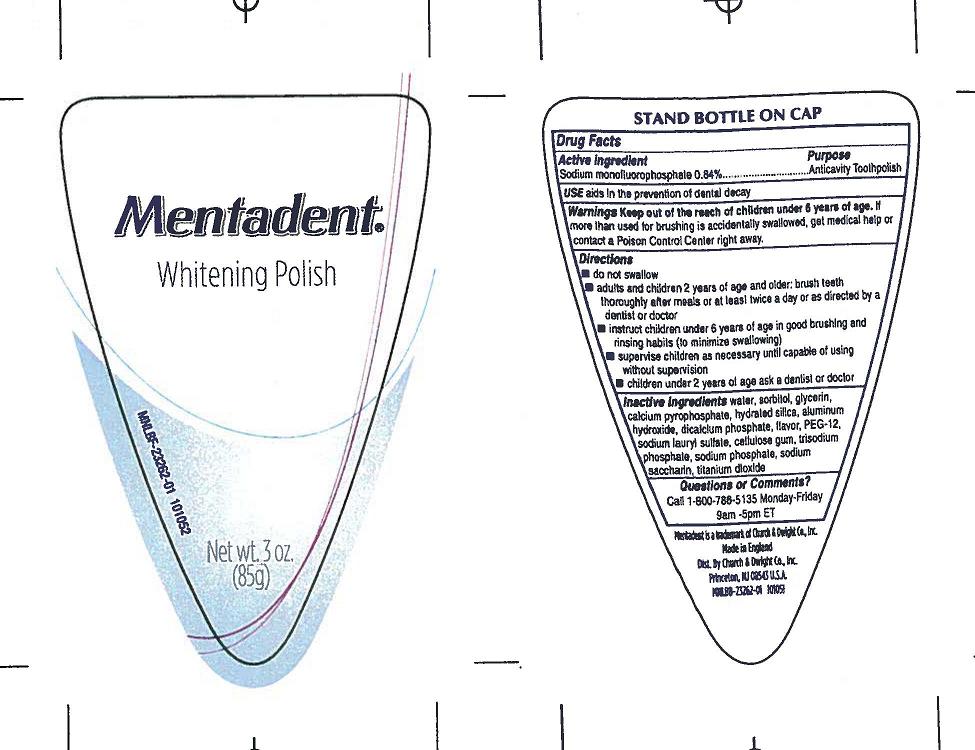
| MENTADENT TOOTHPOLISH sodium monofluorophosphate gel, dentifrice | ||||||||||||
| ||||||||||||



