Dosage form: capsule, extended release
Drug class:5-aminosalicylates
Medically reviewed by Drugs.com. Last updated on May 1, 2022.
On This Page
Mesalamine Controlled-Release Capsules Description
Mesalamine extended-release capsules, USP for oral administration are extended-release formulation of mesalamine, USP, an aminosalicylate anti-inflammatory agent for gastrointestinal use.
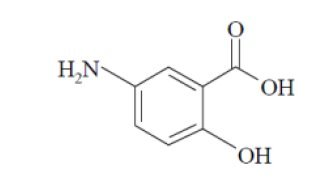
Chemically, mesalamine, USP is benzoic acid, 5-amino-2-hydroxy. It has a molecular weight of 153.14.
The structural formula is:
Each 500 mg mesalamine extended-release capsule contains 500 mg of mesalamine, USP. It also contains the following inactive ingredients: castor oil, colloidal silicon dioxide, diacetylated monoglyceride, ethylcellulose, hypromellose, stearic acid, sugar spheres (corn starch and sucrose), talc, and white wax. The capsule shell contains FD&C Blue 1, gelatin, sodium lauryl sulfate, and titanium dioxide. The imprinting ink contains ferrosoferric oxide, potassium hydroxide, and shellac.
FDA approved dissolution test specifications differ from USP.
CLINICAL PHARMACOLOGY
Sulfasalazine is split by bacteri...